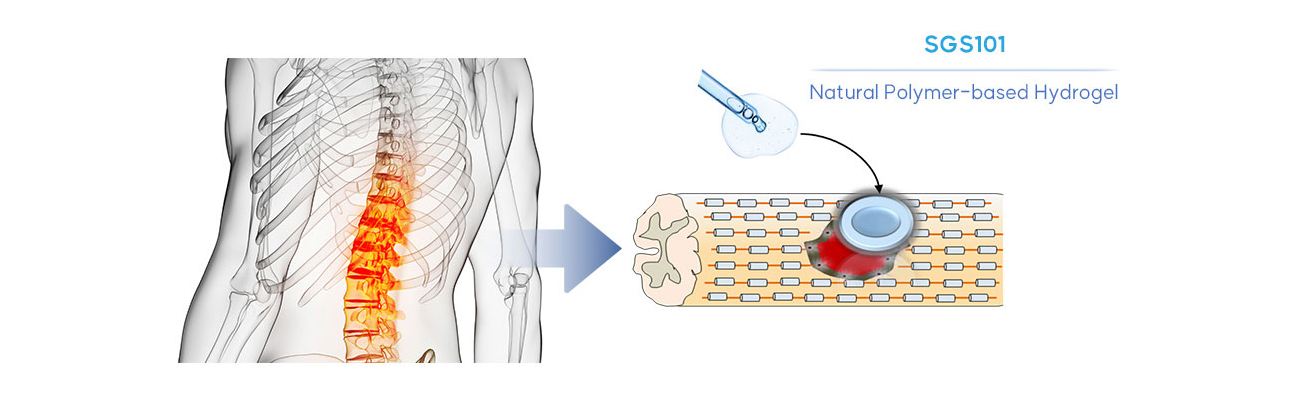
Spinal Cord Injury Therapeutics
Natural Polymer-based Hydrogel(SGS101)

- First-in-class : Therapeutics for spinal cord injury
- Biological safety : ECM-based natural polymers
- Drug repositioning/drug repurposing : Composition of 3 FDA-approved ingredients
- No toxicity : Preclinical toxicology studies was performed by Good Laboratory Practice (GLP) facility.
- Best-in-class : Remarkable therapeutic efficacy
- New MOA : Optimization of tissue micro-environment for regeneration
- Golden time: Applicable immediately after injury
- Non-invasive treatment : Applicable without additional surgical process
The primary target condition of SGS101 is acute traumatic spinal cord injury (SCI). Typical surgical techniques are ineffective to cover the injured and lost tissue, therefore, there is no cure for fundamental treatment for SCI. SuPine Therapeutics developed ECM-based natural polymers for spinal cord injury treatment (SGS101). SGS101 is intended for topical use providing the protection of injury site and support recovery. SGS101 can affect throughout the healing process as a barrier to protect the wound on spinal cord. SGS101 blocks the secondary injury.
Data
Superior therapeutic effects on motor function recovery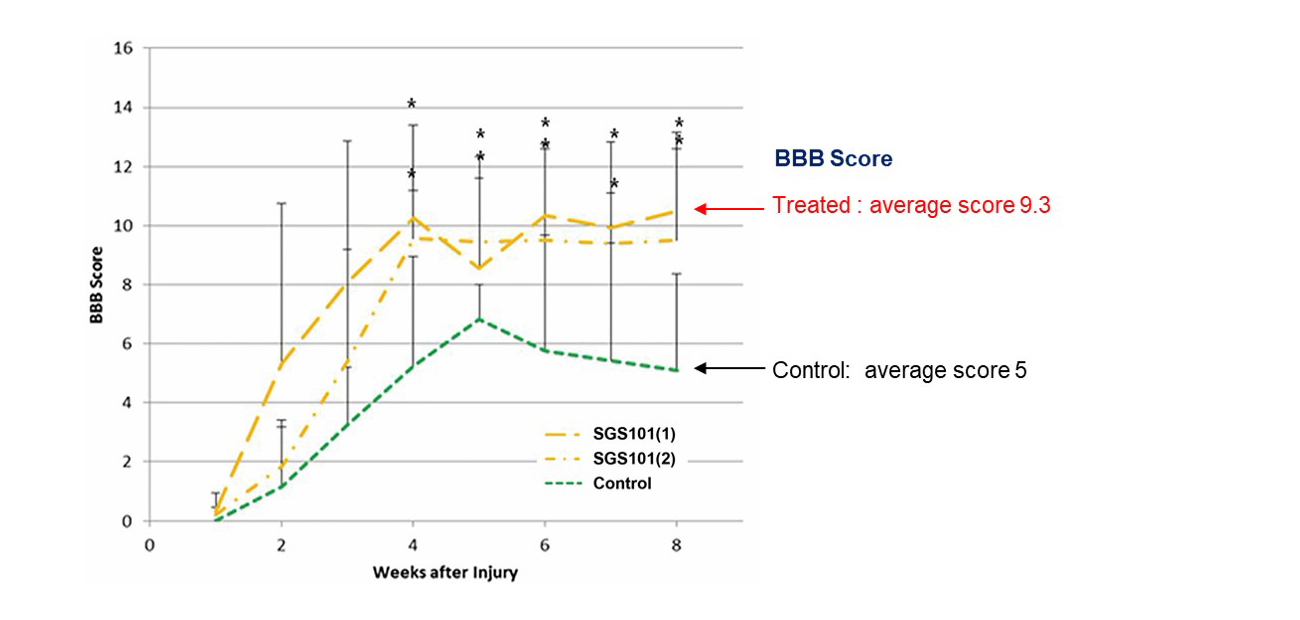
BBB(Basso, Beattie and Bresnahan) score indicates motor function of hindlimb. Transplantation of SGS101 significantly increased motor function recovery in rat spinal cord injury model.
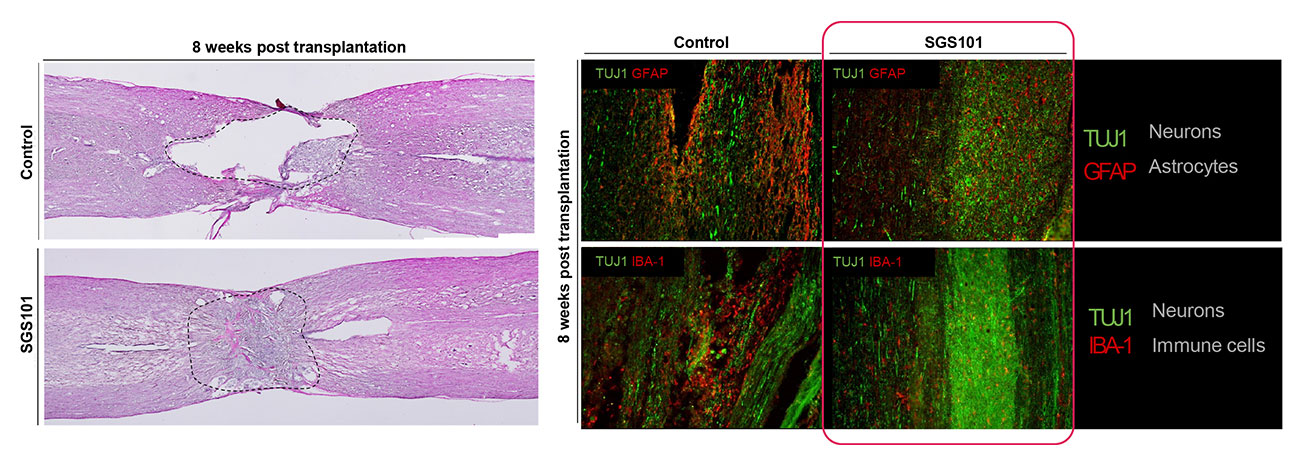
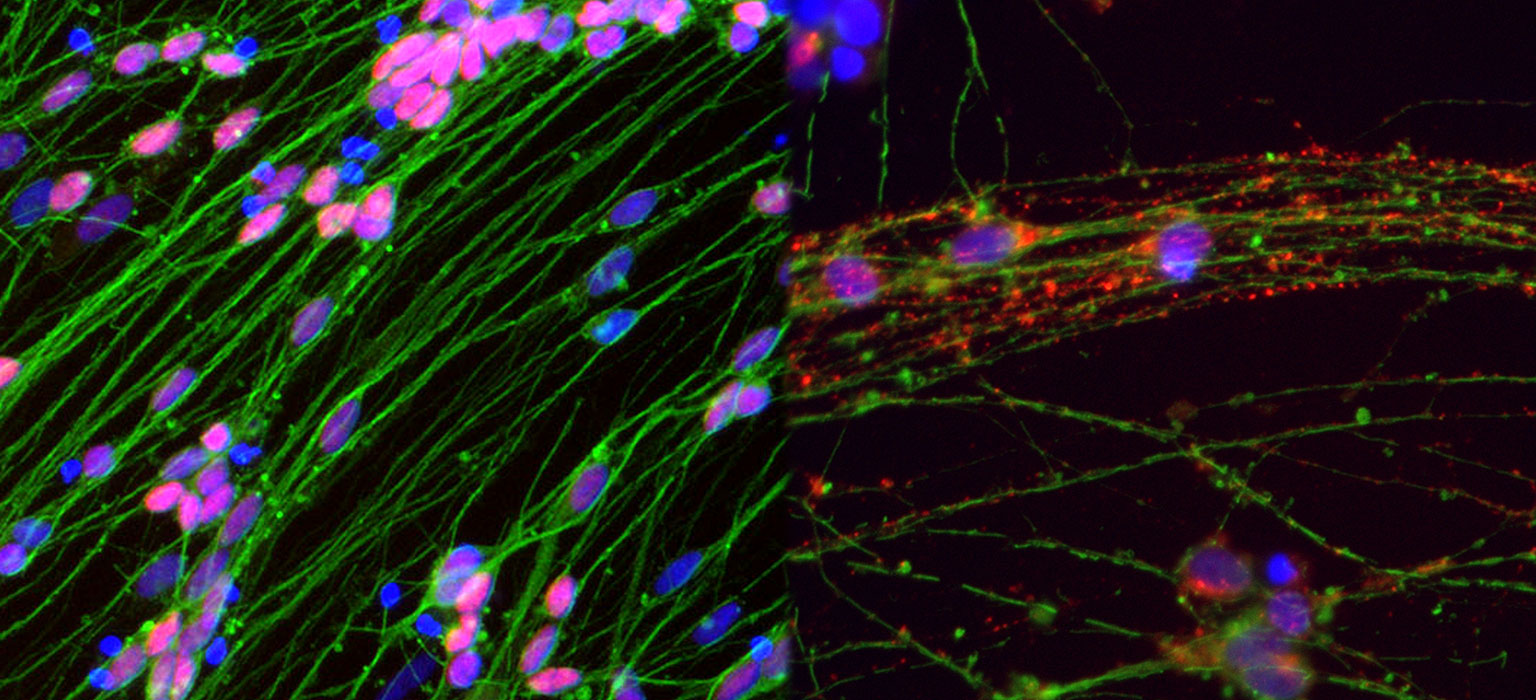
The neural tissue regeneration was confirmed. The cavity size was significantly reduced in SGS101 treated group compared to untreated group. The number of immune cells and glial scar-forming astrocytes were decreased at the injury site in SGS101 treated spinal cord injury model.
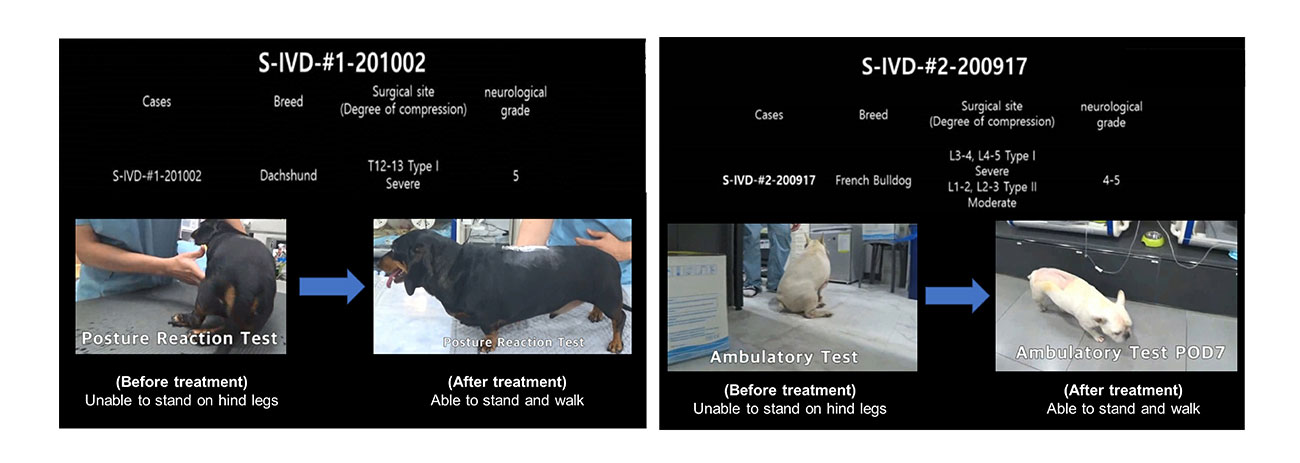
The efficacy in mid-sized animal was confirmed by canine pre-clinical study. Motor and sensory functions were improved and recovered significantly within 2 weeks in 4 canines.
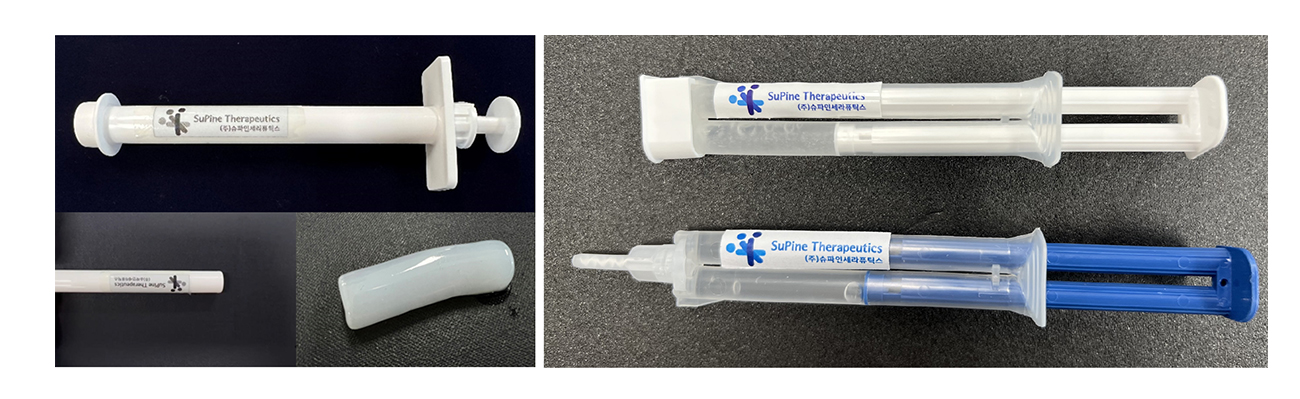
SuPine Therapeutics expanded intended use of SGS101 to treat pets with neural injury caused by disc herniation. Our veterinary medical device (SGD101) is designated as a deep-cavity wound dressing. We target the veterinary medical market.





